by Arnaud Divialle
Plastic injection molds are complex heat exchangers
This primer on the thermal physics of plastic injection will help you understand the complex thermal energy at play during plastic injection molding and help you design better molds.
The magic of plastic injection
The magic of plastic injection is that it transforms molten resin into a solid plastic piece.
The mold’s job is to cool the resin quickly and uniformly, ensuring high part quality and cost efficiency.
This is accomplished mainly during the mold cooling phase, when heat energy is transferred from the molten resin to the metal of the mold and then to the coolant liquid.
What is heat?
Heat is a thermal form of energy. At the micro level, heat is the vibration of particles – the kinetic energy of atoms and molecules.
As with any form of energy, heat cannot be created or destroyed, only transferred or transformed.
Heat sources in plastic injection
Three sources of heat exist in the plastic injection process:
- The main source is the temperature of the molten resin.
- Some heat comes from shear heating as the resin flows into the mold (meaning the kinetic energy of the flow is converted into thermal energy).
- And, in some cases (where heat and cold or silcone are involved, for example), the mold is heated, thereby adding thermal energy via mold temperature.
How is heat transferred?
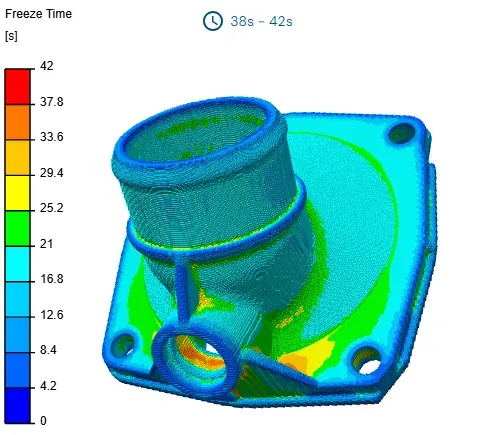
The first rule is that heat always moves from areas of higher temperature (high energy) to areas of lower temperature.
In the context of injection molding, there are two main types of heat transfer for thermal energy: conduction and convection.
Conduction
Conduction is the transfer of heat within a body or between bodies (whether in solid, liquid or gas form) that are in direct contact. It requires no movement of material.
Conduction heat transfer happens inside the mold during the cooling phase. The heat flows, by conduction, inside the metal, from the cavity surface to the colder regions of the mold (mostly toward the water lines), without material movement.
In conduction, a fast vibrating atom (one with a lot of kinetic energy, meaning high temperature) drags a neighboring atom along with it, thereby transferring some of its kinetic energy. This only happens if the neighboring atom has less energy (a lower temperature). Because atoms vibrate within their particles, no material flow occurs. Absent any external source of energy, heat passes from atom to atom until they all vibrate at the same level, creating a uniform temperature within the material.
The flow of thermal energy – how quickly the temperature changes throughout the material and over time – is influenced by four factors:
- Temperature gradient: The larger the temperature difference, that faster the heat will flow, and the more readily a high-temperature particle will share its energy with neighboring particles.
- Material thermal conductivity: Thermal conductivity is a property related to the material’s microstructure and density, meaning how much it can vibrate and how closely packed the atoms are in the material. The closer they are, the easier it is for energy to be transferred between them.
- Duration: With more time, more heat can transferred. This parameter – “cooling time” – is one of the easiest to adjust, and is the main concern in injection molding.
- Geometry of the medium: The larger the area that the heat has to come through, the easier it will be. With a longer heat path, the thermal energy will flow more slowly.
Convection
Convection is the transfer of energy by the movement of fluids. Convection can either be free or forced depending on the how the movement of the fluid is generated.
Free convection
This is what happens at the surface of the mold, where it is in contact with the air in the shop.
- In free convection, the fluid movement is driven by the thermal phenomena alone. When a pocket of fluid is in contact with a hot section, heat flows from the hot section to the fluid particles by conduction (cooling the hot section as aresult).
- As the temperature of a fluid increases, the vibration of the atoms also increases, and the atoms need more space to move. As a result, they move farther away from each other. This increase in distance between atoms leads to an increase in volume, meaning that the fluid density decreases.
- As density decreases, the fluid becomes less susceptible to gravity and rises. In rising, the pocket of hot fluid pushes the fluid above it, which tends to create a void below it, close to the heating region. The process is repeated as colder pockets of fluid take its place in contact with the hot section until they also heat up and push upwards.
- As they rise, the fluid pockets move away from the heating region and tend to cool down (in part by conduction). In cooling, they get heavier (their molecules need less space to vibrate) and drop, and some get closer to the heating element, initiating fluid circulation. Free convection effectively cools the heating element. But, as the fluid moves away from the heat source due only to its buoyancy, the heat transfer process is quite slow.
Forced convection
This is what takes place inside the water lines. Water is put into contact with hot sections of the mold and extracts the heat. The water flow, forced by the chiller pump, continually carries away heated pockets of water and replaces them with colder water.
- To increase the rate at which heat is extracted, convection can be forced by a fan or a pump. The hot section continually comes into contact with a new pocket of cold fluid. Due to the temperature difference, heat moves quickly from the heating element to the fluid. The heated pocket is carried away, and the heating element is exposed to a new cold pocket. As the heated element is continuously exposed to cold pockets of water, the heat transfer is much faster than with free convection.
- Heat transfer in convection is driven by the same principles as in conduction, with the addition of fluid movement (flow rate). Heat flux is characterized by a heat transfer coefficient that accounts for the materials and geometries involved, as well as for the fluid flow behavior. Those coefficients are empirical and listed in authoritative publications¹.
How does the choice of material affect the flow?
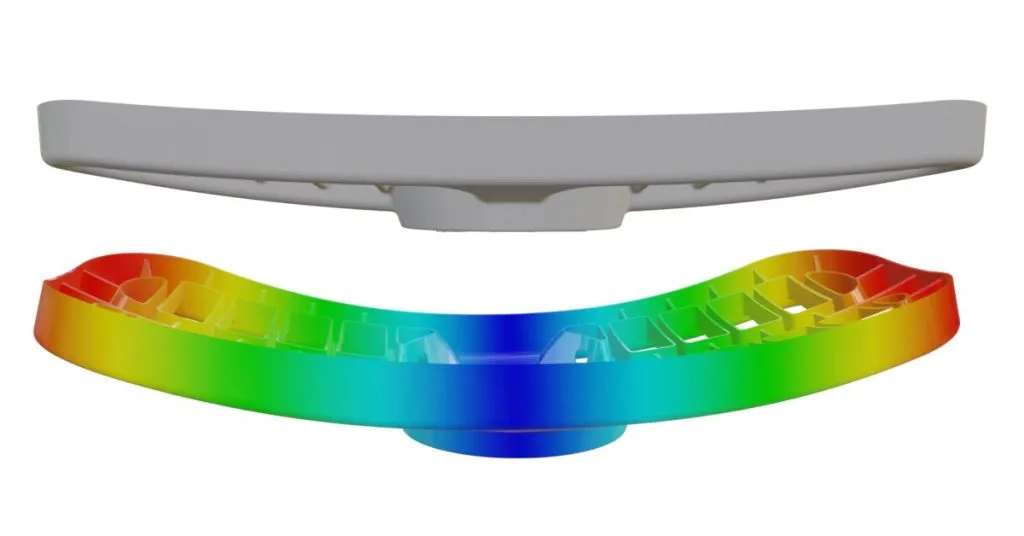
The properties and characteristics of materials have an effect on the flow.
Heat capacity
A material’s heat capacity is its ability to store heat.
- It describes the amount of additional energy needed to increase the temperature of a given volume of material by 1 degree. The heat capacity of a material changes with its state (solid, liquid, gas). Heat capacity is expressed as J/K or BTU/˚F in the international and British systems, respectively.
- Imagine two pots full of water, one small and one large, on a stovetop. The large pot would take longer to heat up because it can hold more heat. Similarly, materials with a high heat capacity require more added heat to increase their temperature.
- In plastic injection, crystalline resins (e.g., PE or PP) usually have a higher heat capacity that amorphous resins (e.g., PS). Therefore, crystalline plastics contain more heat than amorphous resins, and release it slower. Metals have much lower heat capacity than plastics, making them better at transferring heat rather than holding it.
Thermal conductivity
A material’s thermal conductivity is its ability to transfer heat.
- It describes how far and how fast heat will flow in a given material. Thermal conductivity is expressed as W/m.˚C or BTU/hr.f.˚F in the international and British systems, respectively. Metal has a much greater thermal conductivity than plastic.
- Consider how it feels to touch a cold object in a cold environment. Touching it with a bare hand transfers a portion of your body heat to the material. If the material is colder than your hand, and if it has a high thermal conductivity as metal does, the heat will quickly be removed. The temperature of your skin will tend toward balancing out with the rest of the (cold) material, and your hand will feel cold. If your hand transfers more heat than what the material can evacuate (if the material has low thermal conductivity), then the temperature of that part will increase and feel warm.
- In general, plastics have a (much) lower thermal conductivity than metals. Copper and aluminum, in particular, have high thermal conductivity, which is why they’re used for heat sinks. Materials with low thermal conductivity are more often used as insulators. Where copper has a thermal conductivity of around 400 W/m·K, a plastic like polypropylene is roughly 1000 to 1300 times less conductive, with a range of 0.1-0.3 W/m·K.
Density
A material’s density is how many particles it stacks per given unit of volume. It is expressed as kg/m3 or lbs/in3 in the international and British systems, respectively.
- Density is related to mechanical properties and thermal properties. In particular, density is directly tied to thermal conductivity. The closer the particles are together (higher density), the more readily they share their kinetic vibration energy (heat), meaning the thermal conductivity is higher.
Specific heat
A material’s specific heat is its ability to store heat, taking its weight into consideration. It describes the amount of additional energy needed to increase the temperature of 1 kg or 1 lb of the material by 1 degree. Quite simply, it is heat capacity divided by the density. Specific heat is expressed as J/K.kg or BTU/˚F.lbs in the international and British systems, respectively.
Material properties are dependent on temperature
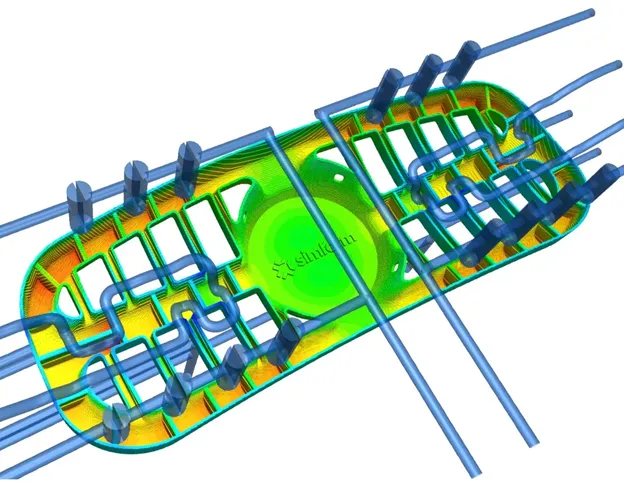
A material’s heat transfer properties vary significantly depending on its state (solid, liquid, gas). During the plastic injection process, as the plastic changes state, so do the material properties of the resin, according to its molecular structure (amorphous, crystalline or semi-crystalline).
- Crystalline polymers (such as PEEK or PET) have a strictly ordered molecular structure and a clearly defined melting point that distinguishes under which conditions the material behaves as a fluid or as a solid.
- Amorphous polymers (such as PMMA Acrylic, Polystyrene and Polycarbonate) have a random molecular structure. Their properties and behaviours gradually change from rubber-like to glass-like. The transition point is called the Gla
- Semi-crystalline resins are a structural and behavioral cross between crystalline polymers and amorphous polymers.
Given that the objective of the plastic injection process is to solidify the resin, it’s important to define the target temperature. It’s a critical parameter in the injection manufacturing process. Usually referred to as the safe ejection temperature, it might be based on the Heat Deflection temperature or on the Glass Transition Temperature, depending on the resin structure.
Thermal properties of key materials used in plastic injection
There is a noteworthy difference in the thermal conductivity between metals and plastics – and it explains why heat struggles to leave the plastic part whereas it quickly flows through the mold material.
| Material | Density (g/cm³) | Thermal Conductivity (W/m.K) | Heat Capacity (J/kg.K) | Specific Heat (J/g.K) |
| P20 Tool Steel | 7.85 | 41.5 | 470 | 0.47 |
| H13 Tool Steel | 7.8 | 29 | 470 | 0.47 |
| Moldmax | 8.4 | 105 | 380 | 0.38 |
| 13-8Mo Stainless Steel | 7.8 | 16 | 500 | 0.5 |
| 6000 Aluminum | 2.7 | 167 | 897 | 0.9 |
| ABS | 1.04 | 0.17 | 1,800 | 1.8 |
| PMMA (Acrylic) | 1.18 | 0.19 | 1,470 | 1.47 |
| Nylon (PA6) | 1.14 | 0.25 | 1,700 | 1.7 |
| Polycarbonate (PC) | 1.2 | 0.2 | 1,200 | 1.2 |
| Low Density Polyethylene (LDPE) | 0.92 | 0.33 | 2,300 | 2.3 |
| High Density Polyethylene (HDPE) | 0.95 | 0.5 | 1,900 | 1.9 |
| Polypropylene | 0.9 | 0.22 | 1,900 | 1.9 |
| Water | 1.0 | 0.6 | 4,181 | 4.2 |
Material selection has a major impact on heat flow and mold cooling
The heat transfer problem may seem simple:
- Heat from the resin is transferred through the resin and the mold by conduction.
- It is then extracted by the water lines by forced convection.
However, it becomes dramatically more complex when a mold has multiple components of complex geometries and is made from different materials with properties that vary with temperature.
In fact, the problem is so complex that simple approximations and 1D or even 2D models can't accurately predict the heat flow throughout the injection molding process.
It’s an important problem to solve because these thermal factors drive part quality and cost.
More advanced solutions are needed to properly estimate the thermal behavior of the mold.
How SimForm delivers solutions
The fast and user-friendly simulation tools in SimForm help mold designers to frontload simulation in the mold design process and better visualize the heat flow within their molds.
SimForm accounts for almost all aspects of heat transfer problems – actual part geometry, actual mold geometry and the material properties of all the components involved – and then simulates the heat conduction within the mold and its convection (both free convection with the shop environment and forced convection in the mold cooling lines).
The result is a much more accurate prediction of temperature distribution and cooling time than can be obtained with 1D and 2D models.
SimForm also works much faster than most 3D mold flow simulation tools in a cloud-based format that gives mold makers the best balance of accuracy and speed.
For mold designers, SimForm is a critical tool for visualizing the thermal performance of their molds and developing better performing molds faster.
Discover the benefits of SimForm for your molding.
References
1. Dittus, F. W. and Boelter, L. M. K., Heat transfer in automobile radiators of the tubular type. University of California Publications in Engineering, 1930